Description
Powder and solvent for solution for injection.
After reconstitution, 1 dose (0.5 mL) contains: Neisseria meningitidis serogroup A polysaccharide* 5 micrograms, Neisseria meningitidis serogroup C polysaccharide* 5 micrograms, Neisseria meningitidis serogroup W-135 polysaccharide* 5 micrograms, Neisseria meningitidis serogroup Y polysaccharide* 5 micrograms, *conjugated to tetanus toxoid carrier protein 44 micrograms.
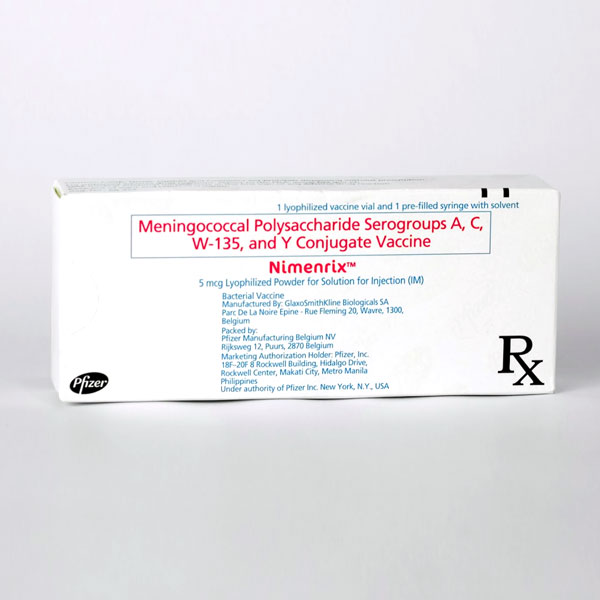
Meningococcal polysaccharide serogroups A, C, W-135 and Y conjugate vaccine (Nimenrix) is composed of the purified capsular polysaccharides of Neisseria meningitides types A, C, W and Y, each conjugated to tetanus toxoid (toxoid to polysaccharide).
Meningococcal polysaccharide serogroups A, C, W-135 and Y conjugate vaccine (Nimenrix) is a non adsorbed freeze-dried preparation presented as a monodose vial to be reconstituted with a diluent (0.9% sodium chloride solution). The final reconstituted vaccine is administered through intramuscular injection.
Excipients/Inactive Ingredients: Powder: Sucrose, Trometamol.
Solvent: Sodium chloride, Water for injections.