Description
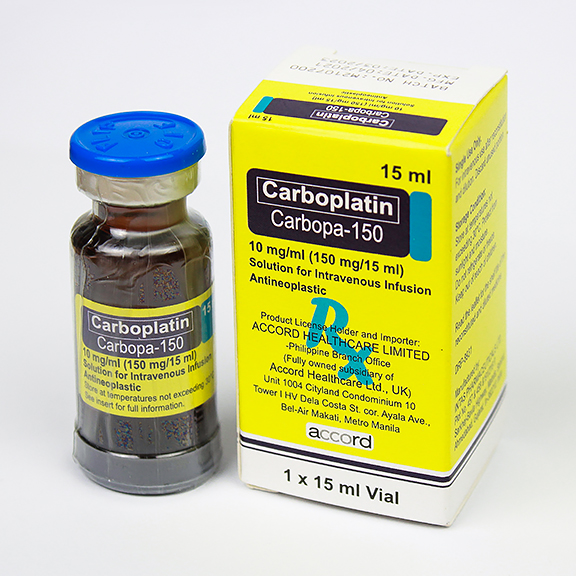
Contents: Carboplatin
Indications/Uses: Treatment of advanced stage ovarian cancer of epithelial origin.
Dosage/Direction for Use
Previously untreated adult (w/ normal renal function) 400 mg/m2 by IV infusion over 15 min to 1 hr. Doses should not be given more frequently than every 4 wk. Renal failure CrCl 41-56 mL/min Initially 250 mg/m2. CrCl 16-40 mL/min Initially 200 mg/m2.
Contraindications
Hypersensitivity to carboplatin or other platinum containing compd. Severe myelosuppression; bleeding tumours; pre-existing severe renal impairment (CrCl ≤20 mL/min). Breast-feeding.
Special Precautions
Patients w/ abnormal kidney function or receiving concomitant therapy w/ other drugs w/ nephrotoxic potential. Assess carefully renal function parameters before & during therapy. Infusion courses should not be repeated more frequently than mthly under normal circumstances. Monitor peripheral blood counts frequently. Combination therapy w/ other myelosuppressive compds. Supportive transfusional therapy may be required in patients who suffer severe myelosuppression. May cause nausea & vomiting. Renal & hepatic function impairment. Increased incidence of allergic reactions w/ previous exposure to platinum therapy. Perform neurological evaluation & assessment of hearing on a regular basis especially when receiving high dose. Do not use Al containing equipment during prep & administration. Women of childbearing potential & pregnancy.