Description
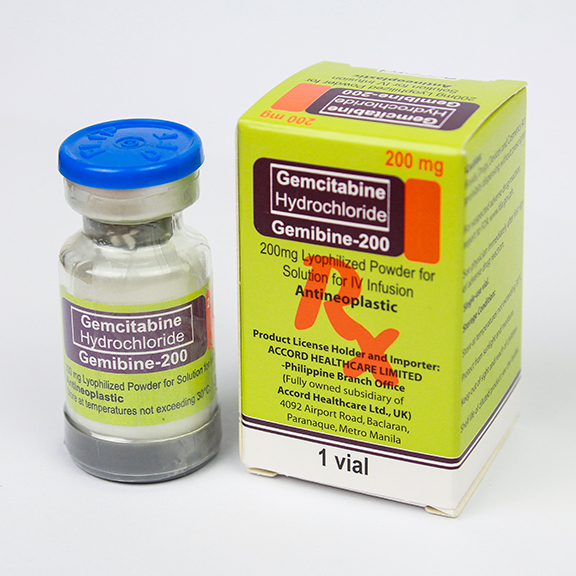
Gemcitabine for Injection USP 200 mg
Each vial contains:
Gemcitabine Hydrochloride Ph.Eur.
equivalent to Gemcitabine 200 mg
Excipients: Q.S.
Therapeutic indications
Gemcitabine is indicated for the treatment of advanced bladder cancer (muscle invasive Stage IV tumours with or without metastases) in combination with cisplatin.
Gemcitabine is indicated for treatment of adult patients with locally advanced or metastatic adenocarcinoma of the pancreas.
Gemcitabine, in combination with cisplatin is indicated as first line treatment of patients with locally advanced (inoperable Stage IIIA or IIIB) or metastatic (Stage IV) non-small cell lung cancer (NSCLC). Gemcitabine is indicated for the palliative treatment of adult patients with locally advanced or metastatic NSCLC.