Description
For intramuscular use only.
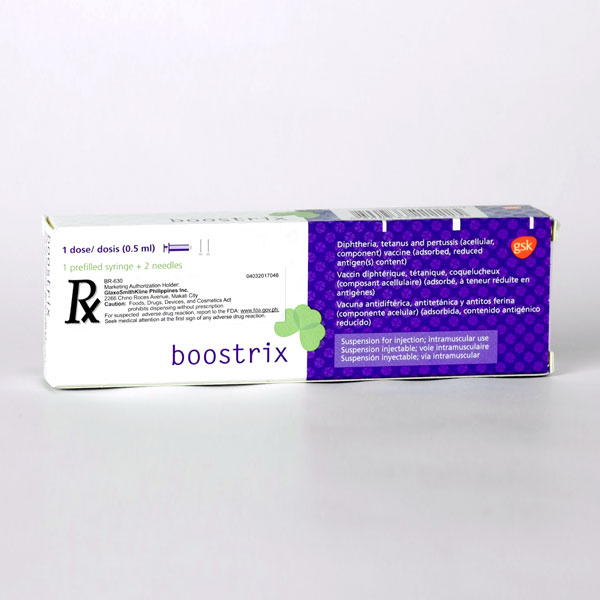
• Each dose of BOOSTRIX is administered as a 0.5-mL injection. (2.2)
• An initial dose of BOOSTRIX is administered 5 years or more after the last dose of the Diphtheria and Tetanus Toxoids and Acellular Pertussis (DTaP) series or 5 years or more after a dose of Tetanus and Diphtheria Toxoids Adsorbed (Td). BOOSTRIX may be administered as an additional dose 9 years or more after the initial dose of Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine Adsorbed (Tdap). (2.2)
• BOOSTRIX may be administered for tetanus prophylaxis for wound management. For management of a tetanus-prone wound, a dose of BOOSTRIX may be administered if at least 5 years have elapsed since previous receipt of a tetanus toxoid-containing vaccine. (2.2)